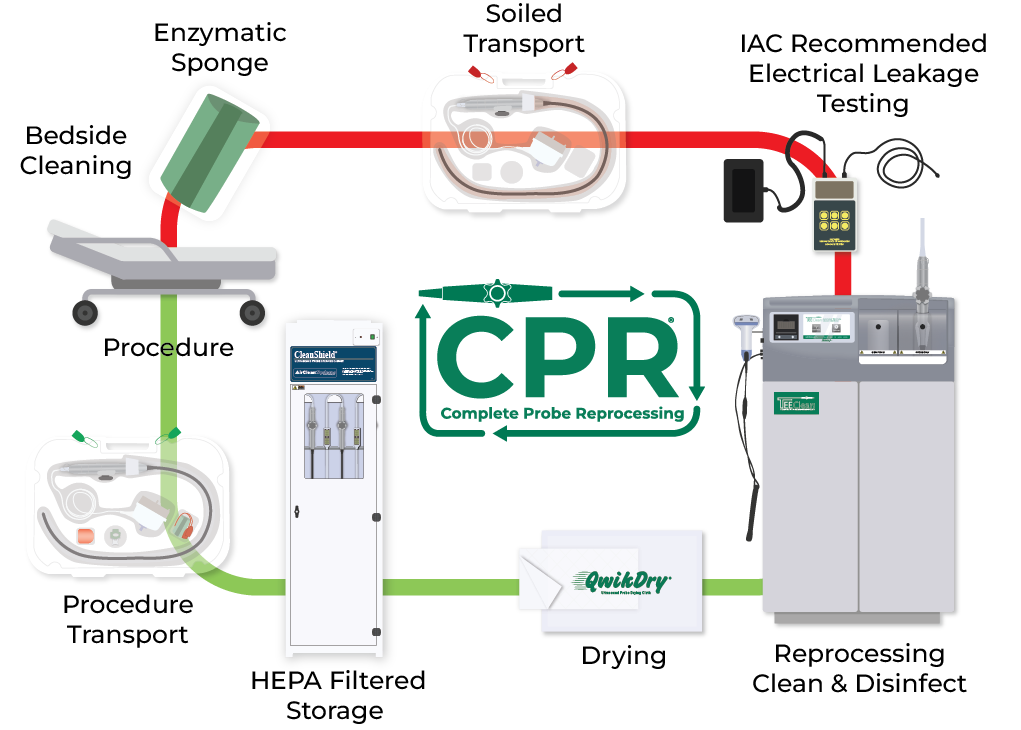
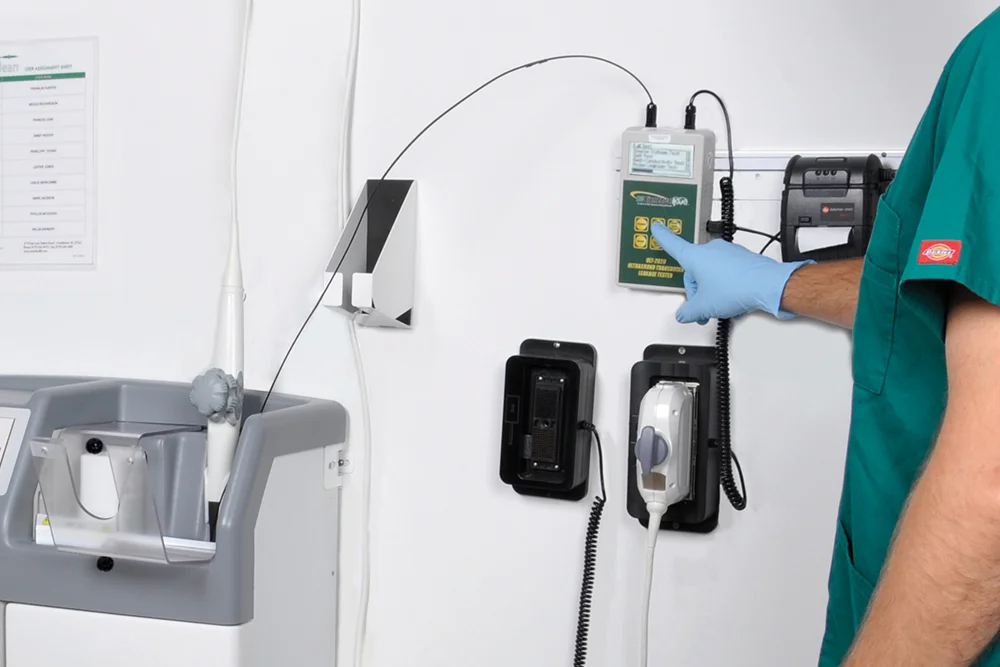
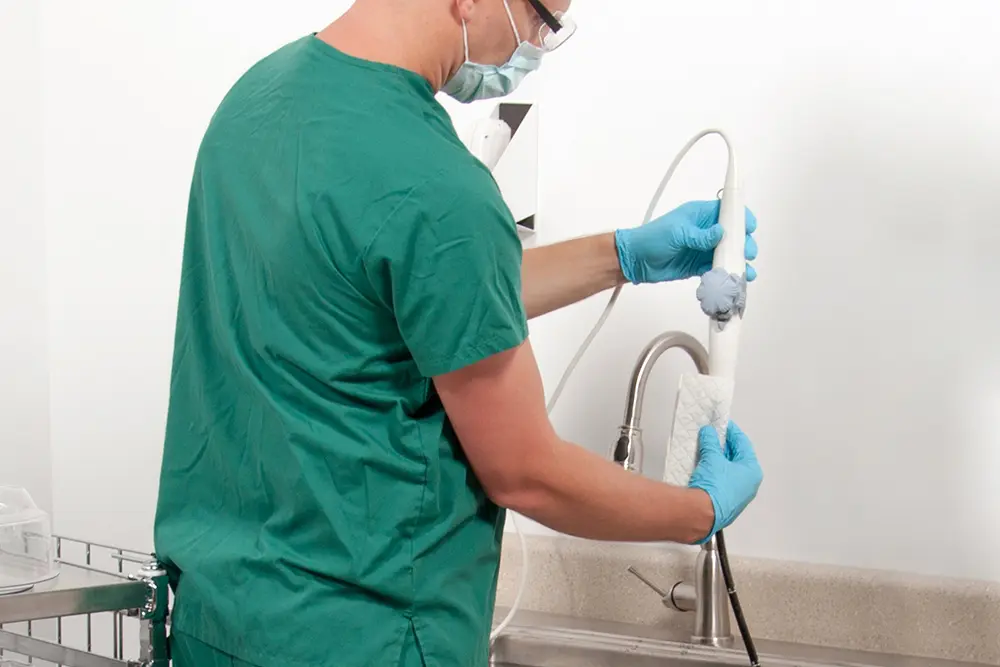
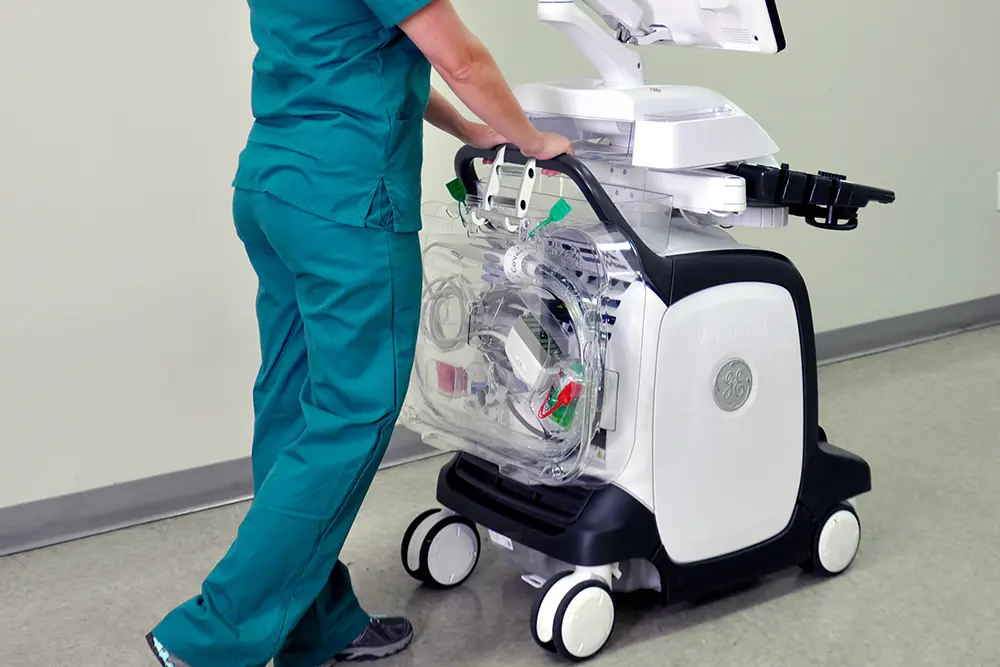
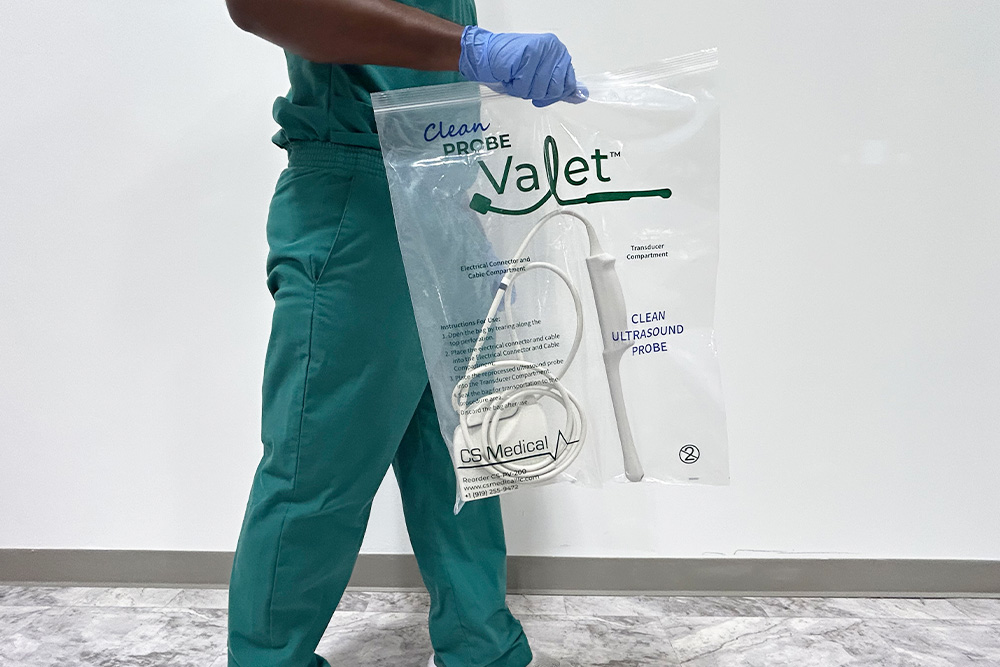
A standardized, evidence-based guidelines approach to the reprocessing of TEE ultrasound probes improves quality, reduces variations, and controls cost in a variety of areas, from patient care to supply chain to overall departmental revenue management.