


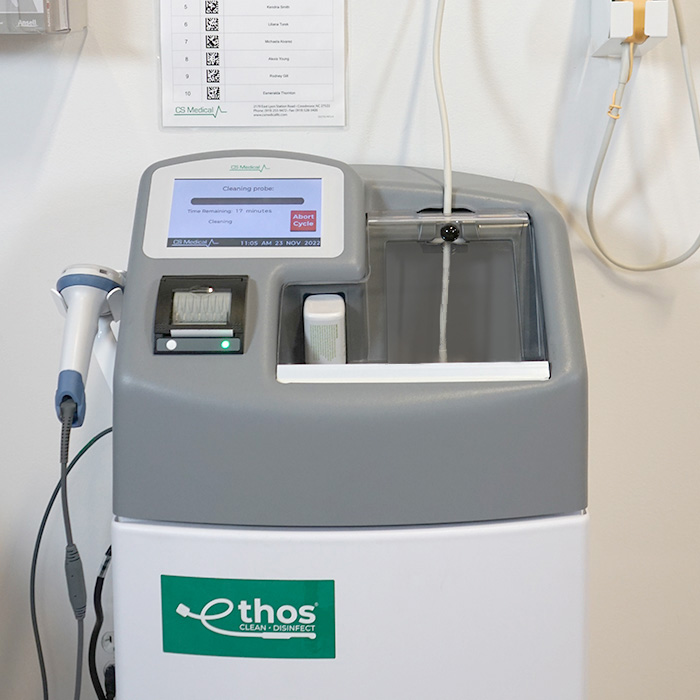



Ethos Automated Ultrasound Probe Cleaner Disinfector is the first product of its kind to be cleared by the US FDA. Ethos provides both manufacturers recommended cleaning and high-level disinfection of soiled transvaginal, transrectal, and surface ultrasound probes in the same device. Ethos is approved for use with Philips, Samsung, and Siemens ultrasound probes.
Ethos automated cleaner disinfector is fast and easy to use. The soiled probe must have the procedure cover removed prior to direct placement into Ethos, no additional steps are required to prepare the probe before it is placed into the device. Then, by following simple prompts on the color LCD display, the healthcare professional will scan information about the probe, disinfectant lot and their user ID. This data, in addition to other system data, will be printed on the reprocessing report at the conclusion of a completed cleaning and disinfection cycle.
For the healthcare facility and technician, Ethos removes the unknowns that exist with manual cleaning, manually reading of a chemical indicator strip or chip, and provides an added level of confidence that each endocavity or surface probe receives the same care during cleaning and disinfection every cycle.






