TEE Probe Overview
Knowing the TEE Ultrasound Probe
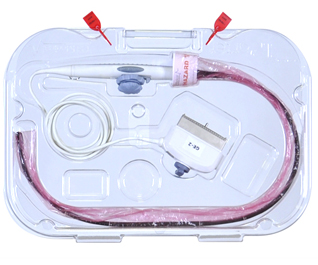
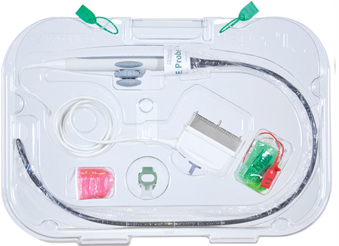
TEE ultrasound probes consist of five main parts, as described by probe manufacturers and as illustrated above
The electrical connector (4) connects the ultrasound machine to the probe.
The cable (3) of the probe connects to the handset (5) to the electircal connector. This steering mechaism and handle are not water tight and should not be submersed in liquid.
The long insertion tube (1) with transducer at the distal tip (2) can be difficult to handle during pre-cleaning, cleaning, high-level disinfection, drying and subsequent storage and transportation.
The Challenges
The overall length of the TEE ultrasound probe and the fragile nature of the transducer make handling of the device susceptible to damage and challenging for healthcare professional. Care to minimize excessive handling and contact shock to the distal tip should be taken when manipulating the components during all facets of reprocessing. To properly reprocess a TEE probe the technician should be aware of the delicate nature of various components.
The critical difference between a colonscope or a gastroscope and a TEE probe is it can't be completely submerged into the rise and high-level disinfectant baths. The TEE probe is not water tight and complete submersion of the probe could cause serious damage and result in the inoperability of the TEE probe.
A thorough understanding of the TEE ultrasound probe and how it should be reprocessed is critical. This knowledge will lead to minimized TEE ultrasound probe damage, improved patient outcome and allow for proper reprocessing that minimize Healthcare-Associated Infections (HAIs)
Why Infections Happen as it relates to TEE reprocessing
When the TEE probe is removed from the patient the "golden hour" begins. The "golden hour", as described by many cardiovascular doctors and infection control coordinators, is the time between when the probe leaves the procedure room to be reprocessed and it is returned to be used on the next patient.
During the "golden hour" the first instance where a mistake could lead to an improperly reprocessed TEE probe occurs right at the point of use, the patients' bedside. The use of a sponge impregnated with an enzymatic detergent to wipe down the probe immediately after a procedure is imperative. Allowing bioburden to dry on the sheath of the probe will make it harder for the technician to remove and will result in longer turnaround time or the probe being reprocessed incorrectly. Biofilm is an issue that must be addressed as it can effectively envelop various containments and prevent the exposure of such containments to the high-level disinfectant. This would result in a TEE probe being returned to the procedure room, for a patient, that has not been properly reprocessed. Bedside treatment of the probe is step one. It is critical to ensuring the TEE probe is receiving the proper care and getting reprocessed effectively.
Getting the enzymatically laden TEE probe from the patients' bedside to the area where cleaning and high-level disinfection occur represents several issues for the healthcare professional. The "dirty" TEE probe now represents a potential biohazard traveling through the facility. The potential for probe damage is higher during the transportation due to inadequate storage containers or the method for transporting the probe. The insertion tube of a TEE probe has several areas of concern when transporting from bedside to the reprocessing suite. Distal tip damage can occur when the probe is hit or bumped into inanimate objects or other portions of the TEE probe, i.e. the electrical connector. Multiple TEE probe manufactures indicate the bending of the insertion tube into a circle with a diameter of less than 0.30 m or 1 foot can result in damage. This condition can be seen when the TEE probe is placed into a container not designed for or adequate for the TEE probe.
The cleaning process is extremely important as it removes inorganic and organic debris that can otherwise remain on the probe's insertion tube and defeat the efficacy of the disinfection. Cleaning incorporates both an enzymatic soak and a thorough rinsing of the enzyme from the probes insertion tube.
Once the probe arrives in the disinfection suite it must then undergo manufacturer directed pre-cleaning steps so as to prepare the probe for high-level disinfection. The pre-cleaning process is typically completed in a basin or sink with tempered water and an enzymatic solution. The probe, insertion tube ONLY, is placed into the enzymatic solution and allowed to soak for a pre-determined time period before being removed, rinse and dried. Depending on space within the reprocessing suite a single basin system will result in added handling of the TEE probe. Once the probe has been enzymatically soaked it must be completely rinsed to ensure the enzyme is not present and could not affect the high-level disinfection process.
A critical consideration, with regards to proper cleaning, for every infection control professional is the frequency of change out of the enzymatic cleaning solution. Is it changed as often as recommended? If the same diluted enzymatic solution is used for multiple probes and then discard at the end of each day what could be the result or the effectiveness of the solution? This practice could create a dangerous situation due to several known and unknown variables and risk. Optimally, the enzymatic solution should be switched out after each case for maximum safety and to lower the risk of cross contamination.
A thorough rinsing of the probe with water after cleaning is very important to ensure that there is no residue left on the probe. One of the biggest misconceptions in probe cleaning is that you can clean and disinfect with one product. There must always be a two-step process. First, remove the bioburden with an enzymatic detergent and second, use a high-level disinfectant on the probe.
Cleaning is one of the most difficult parts of dealing with medical equipment. "It is the most critical reprocessing step," says Thomas Fischer, PhD, Science Director for CS Medical. "It removes more than 99.9 percent of the bioburden from the probe." he points out. "If a probe is not properly cleaned, terminal disinfection may not be possible. Failure to properly clean probes has considerable consequences for both the patient and the healthcare provider. "Additionally," Dr. Fischer goes on to state, "if reprocessing is delayed, that allows for the accumulation and hardening of bioburden. Bioburden can dry into a nearly impervious substance - harder to remove than chewing gum stuck to the bottom of your shoe."

Prior to inserting the TEE probe insertion tube into high-level disinfectant a drying process should occur that removes residual moisture from the probe. Additionally, a low-lint material that easily slides over the insertion tube should be used so as not to add debris into the high-level disinfection process or damage to the outer surface of the insertion tube.

Improper reprocessing may result in probe damage and higher repair costs for the healthcare facility. This can extend into the thousands of dollars. A complete rebuild of a standard TEE probe can exceed $10,000 or depending on the severity of the damage a new TEE probe could be the only viable solution. The failure to properly clean probes can produce a myriad of avoidable consequences that drain resources from effective healthcare delivery and improving patient outcomes.
Preventing Scope-Related Infections
A good science-based step-by-step approach is key.
The first defense is a good offense and in healthcare, that means education. If employees are educated about how to clean and understand why certain steps are essential, they are much less likely to skip or short-change those steps. In an environment that is very dynamic with new technologies, responsibilities, and turnover, ongoing education and training allows your staff to keep current.
CS Medical's Solutions
TEE Complete Care® was developed to aide healthcare professionals during the "golden hour" with a systematic and effective reprocessing process. The components of TEE Complete Care have been tested on all major brands of TEE probes currently available.
Problem 1:
Not applying an enzymatic to the insertion tube right after removal from the patient's mouth.
TEE Complete Care Solution
TEEZyme® Enzymatic Sponges provide a pre-saturated solution to quickly and effectively wipe down the insertion tube of the TEE probe prior to transport to the reprocessing area.
Problem 2:
Transporting a potentially biological hazard through the healthcare facility.
TEE Complete Care Solution
TPorter®® TEE Probe Transportation Case offers the healthcare professional with a dedicated case custom designed to transport TEE probes securely and gives the necessary identification markings for easy visibility by staff of the contents. TPorter is constructed of clear polycarbonate for ease of visibility. The Pull Up™ biohazard sleeve and attachment of red "dirty" tie bands indicate the status of the enclosed probe plus it secures the probe and case during transportation.
Problem 3:
Having the enzymatic sponge bedside to allow for immediate removal of the organic and inorganic material.
TEE Complete Care Solution
TPorter® TEE Probe Procedure Case is a complete delivery system that allows healthcare personnel to move delicate probes throughout the healthcare facility and deliver them in a manner that creates a standardized operating procedure. TPorter provides a standardized method for delivering the necessary components to the procedure room, i.e. TEEZyme Sponge, TEE Probe, PullUp bio-barrier sleeve and the high-level disinfected TEE probe.
Problem 4:
Drying of the TEE ultrasound probe prior to and after high-level disinfection aids in the prevention of contamination.
TEE Complete Care Solution
QwikDry® Ultrasound Probe Drying Cloth is an individually packaged, irradiated cloth with a super absorbent matrix and an ultra-smooth textured surface that effectively removes moisture and slides freely over the TEE ultrasound probe insertion tube. Each cloth is single-use and low-lint, thus removing the potential for cross contamination and potential microbiological growth.
Copyright 2022. All rights reserved. No portion of this document may be reproduced without written permission from CS Medical, LLC