A Case to Automate Both Cleaning and HLD
Reprocessing medical devices is crucial to patient safety and the prevention of healthcare-associated infections (HAIs). A study by The Joint Commission in 2016 found that 74 percent of all immediate threat to life declarations were associated with the improper equipment sterilization or high-level disinfection.1 Similarly, performance of intermediate and high-level disinfection and sterilization of medical equipment, devices, and supplies (IC.02.02.01, EP 2) was recorded as an area with the highest non-compliance amongst facilities surveyed in 2022.(2) Similarly, a survey of infection preventionists in the US found that 20% of respondents were aware of a time that a probe used in a procedure was incorrectly processed.(3) This response rate likely represents an underestimate because deviations from the reprocessing procedure may go unnoticed. Together, these findings suggest that many facilities continue to struggle with proper medical device reprocessing.
Proper medical device reprocessing requires a balance between the time and resources required to complete the procedure and the risk of HAIs and probe damage. Most professional and regulatory agencies recommend that facilities use the Spaulding classification to determine how to reprocess medical devices. This classification system divides medical devices into three categories: non-critical, semi-critical, and critical devices (Table 1). Using this classification system, non-critical devices should be low or intermediate level disinfected, semi-critical devices should be high-level disinfected, and critical devices should be sterilized.(4-8) According to the Spaulding classification, transvaginal and transrectal ultrasound probes are considered semi-critical devices and must be cleaned and high-level disinfected prior to use.(4-8) Importantly, the use of a procedure cover does not change the Spaulding classification of a medical device.(4,5) High-level disinfection (HLD) is a process that eliminates all microorganisms from a device, except for small numbers of bacterial spores. HLD of transvaginal and transrectal ultrasound probes, as well as other medical devices, is no small feat. However, innovations in the last two decades have improved the available transvaginal and transrectal probe reprocessing methods from manual and semi-automated (manual cleaning and automated disinfection) to completely automated procedures.
| Classification | Definition | Recommended reprocessing |
|---|---|---|
| Non-critical | Devices used on intact skin | Cleaning and low- or intermediate- level disinfection |
| Semi-critical | Devices that come in contact with mucous membranes or non-intact skin | Cleaning and high-level disinfection |
| Critical | Devices that come in contact with sterile body sites | Cleaning and sterilization |
Traditional reprocessing has utilized manual cleaning and HLD procedures. In these methods, the transvaginal or transrectal probe is processed at the point-of-use then cleaned and high-level disinfected by submersion in a compatible cleaner and high-level disinfectant, respectively. While these methods do not require specialized equipment, they can be tedious. Each probe must be inspected and reprocessed individually and correctly using a detailed, multi-step procedure that may vary between probe models; potentially resulting in confusion and an increase in human errors. Human error is a significant drawback for any manual procedure. Missteps during the reprocessing procedure can result in ineffective reprocessing putting patients at increased risk of infection and increasing the likelihood of ultrasound probe damage.

In addition to being tedious, manual reprocessing procedures are more time-consuming than their semi-automated (hybrid) or automated counterparts. Using current guidelines and best practices, it is estimated that the total reprocessing time, or the time required to reprocess a probe from point-of-use to storage and hand washing is approximately 48-88 minutes. This timeline is heavily dependent upon the contact time required for the high-level disinfectant. Longer reprocessing times can result in fewer probes available for procedures or require the facilities to purchase additional probes to compensate for longer reprocessing times (Table 2). The manual nature of these methods requires that staff must dedicate the entirety of the lengthy reprocessing time to cleaning and disinfecting a single probe, with little time to complete other clinical duties. Together, these factors may result in pressure on technicians to skip steps to decrease reprocessing times.(9)
| Method | Manual | Semi-Automated** | Automated |
|---|---|---|---|
| Number of Probes | 10 | 12 | 17 |
**Manual cleaning and automated disinfection
Long and tedious manual reprocessing procedures have led to the development of automated disinfectors, which are FDA cleared devices that high-level disinfect ultrasound probes using automated methods. Automated disinfectors can decrease the total reprocessing time as much as 61 minutes. As well as the total operator hands-on time or the time that technicians or clinical staff must spend actively engaged in the reprocessing procedure (estimated 27-35 minutes). However, these procedures still require manual cleaning methods and may depend upon manual testing of the disinfectant concentration. Manual cleaning procedures require dedicated time from the operator to complete and are subject to human error. Further, it can be difficult to clean cracks and crevices using manual cleaning methods; resulting in an improperly cleaned device and suboptimal conditions for high-level disinfection.(5,10,12)
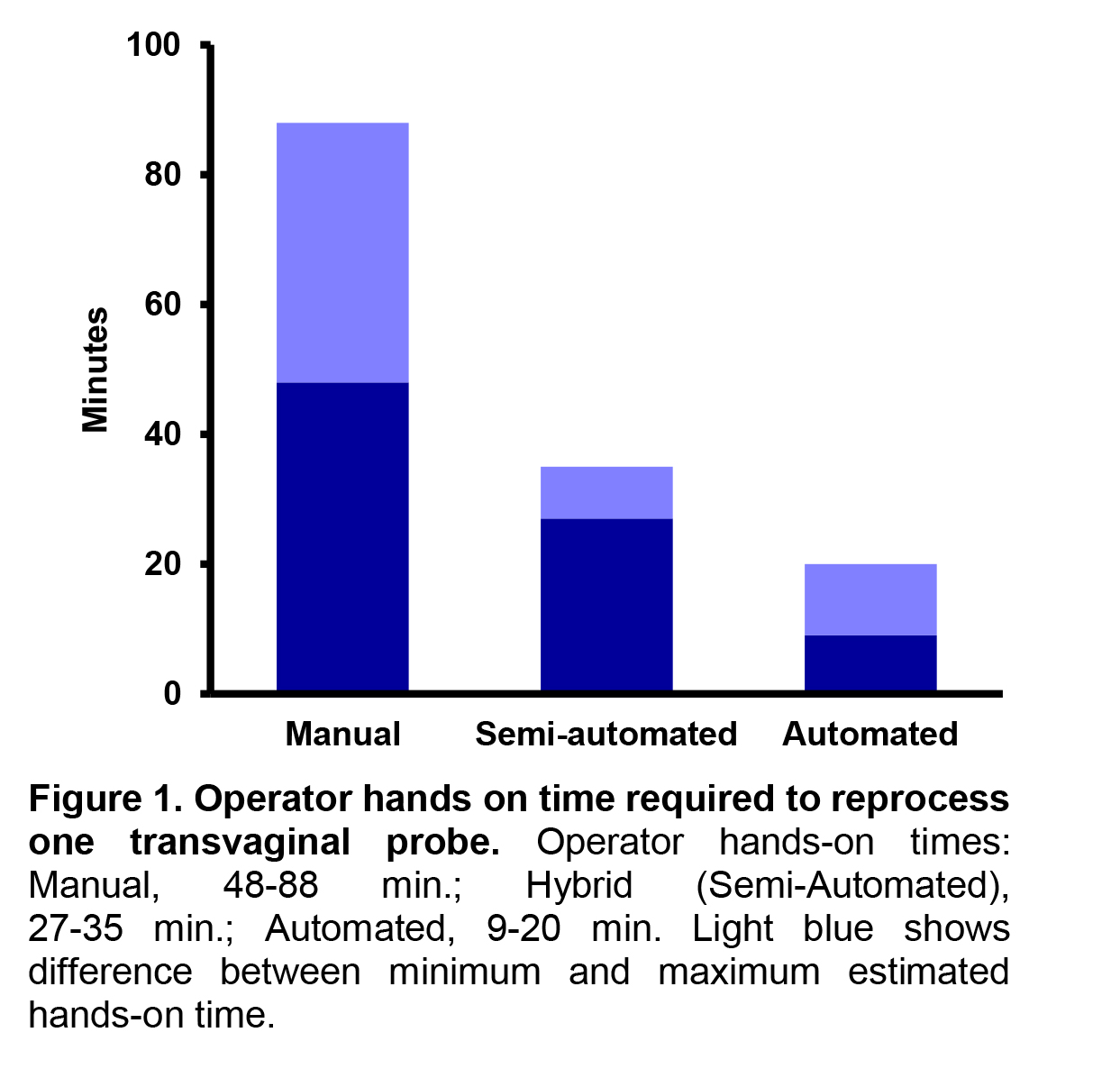
The potential for human error associated with manual methods has resulted in recommendations to use automated methods over manual procedures.(1) Greater than 90% of infection preventionists prefer automated ultrasound reprocessing procedures over manual processes.(3) Similarly, a survey found that staff feel that automated reprocessing procedures are safer than their manual counterparts.(9) However, until recently, there were no options for fully automated reprocessing systems for transvaginal and transrectal ultrasound probes. FDA clearance of the CS Medical Ethos Automated Cleaner Disinfector in 2023 provided the field with the first automated device to clean and disinfect transvaginal and transrectal ultrasound probes in a single cycle. With a total cycle time of 18 minutes, technicians and clinical staff can clean and disinfect probes quickly so they are ready for the next procedure. The automated nature can reduce human errors and the operator hands-on time required for reprocessing to an estimated 9-20 minutes (Figure 1). This leaves at least 18 minutes of each reprocessing cycle available for other clinical responsibilities to maximize staff and technician time. A fully automated reprocessor provides a reliable and repeatable outcome each time and ultimately gives time back to the healthcare professional to perform other critical tasks, like patient care.
Over the last two decades, innovation and technological advances have led transvaginal and transrectal ultrasound probe reprocessing methods from manual to semi-automated, and now completely automated procedures. These changes in methods have also driven changes in regulations and guidance. While there are more options for transvaginal probe reprocessing than ever before, fully automated methods offer numerous advantages. A quick, simple, and efficient workflow can help facilities promote patient safety and prevent HAIs from incorrectly reprocessed ultrasound probes.

Proper cleaning and disinfection of transvaginal and transrectal probes is essential to providing a safe patient environment and preventing healthcare associated infections. Developing and following procedures in accordance with current best practices and guidelines can help reprocessing staff work safely and efficiently without compromising patient safety. CS Medical has developed a guide, “A Complete Users Reprocessing Guide for Ultrasound Probes”(12) to aid healthcare workers in reprocessing transvaginal and transrectal ultrasound probes per current standards and industry guidelines.
Completely automated cleaning and disinfection of transvaginal and transrectal probes in CS Medical’s Ethos automated ultrasound probe cleaner disinfector provides some distinct advantages. Automated workflows are safer for staff by preventing chemical exposures and for patients by reducing the likelihood of human error to provide a reliably cleaned and disinfected probe each time. Further, the ability to use a single reprocessing workflow for compatible probes from different manufacturers can reduce confusion and time spent checking reference materials for each individual probe. Clinical staff and reprocessing technicians have more hands-off time during the automated reprocessing procedure to complete other clinical tasks to maximize their time. The result is a single, efficient, and reproducible workflow that promotes patient safety and infection prevention in healthcare settings.
References
- The Joint Commission, Quick Safety Issue 33: Improperly sterilized or HLD equipment - a growing problem. 2017.
- The Joint Commission, Top 5 Most Challenging Requirements for 2022. 2023, The Joint Commission Online.
- Carrico, R.M., S. Furmanek, and C. English, Ultrasound probe use and reprocessing: Results from a national survey among U.S. infection preventionists. American Journal of Infection Control, 2018. 46(8): p. 913-920.
- Society of Diagnostic Medical Sonography, Sonographer Best Practices for Infection Prevention and Control: Reprocessing the Ultrasound Transducer. 2022: Plano, TX.
- Rutala, W.A., D.J. Weber, and Healthcare Infection Control Practices Advisory Committee, Guideline for Disinfection and Sterilization in Healthcare Facilities, , Centers for Disease Control and Prevention Editor. 2008. p. 14-21. Medicine.
- American Institute of Ultrasound in Medicine, AIUM Official Statement: Guidelines for Cleaning and Preparing External- and Internal-Use Ultrasound Transducers and Equipment Between Patients as Well as Safe Handling and Use of Ultrasound Coupling Gel. J Ultrasound Med, 2023. 42(7): p. E13-E22.
- American College of Emergency Physicians, Guideline for Ultrasound Transducer Cleaning and Disinfection. 2021.
- Food and Drug Administration, Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling, Center for Diagnostic and Radiological Health. 2017
- Johnson, S., et al., Evaluation of a hydrogen peroxide-based system for high-level disinfection of vaginal ultrasound probes. J Ultrasound Med, 2013. 32(10): p. 1799-804.
- Lambert, R.J. and Johnston, M.D., The effect of interfering substances on the disinfection process: a mathematical model. J Appl Microbiol, 2001. 91(3): p. 548-55.
- Rutala, W.A. and Weber, D.J., Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin Microbiol Rev, 1997. 10(4): p. 597-610.
- CS Medical, LLC, A Complete Users Reprocessing Guide for Ultrasound Probes. 2024.